May 8, 2019
Award Sheds Light on Little-Known Achievement
We live our day-to-day lives benefiting from various technologies. And yet we rarely stop to think about how such technologies came about, or what kind of imagination and effort went into their conception. Occasionally an award such as the Nobel Prize will tell us that there’s been a rare discovery. The touch panel on smartphones and other digital devices, for example, began with the development of conductive polymers (in other words, plastics that conduct electricity) by Prof. Hideki Shirakawa, who won the Nobel Prize in Chemistry in 2000. And the 2014 Nobel Prize in Physics popularized the knowledge that LED lighting exists thanks to the development of the blue light emitting diode (LED) by Meidai’s University Professors Isamu Akasaki and Hiroshi Amano.
In other words, the Nobel Prize doesn’t just honor the achievements of researchers. It also tells the world at large that such achievements were behind the technologies many of us use. And it’s not just the Nobel Prize. One effect of awards in general is that they shed light on little-known accomplishments.
The work of Meidai’s University Professor Yoshio Okamoto, who won the Japan Prize this spring, has had a tremendous impact since the early 1980s when his discoveries as an academic researcher were commercialized. Although members of the public don’t come in direct contact with the products, the impact comes from their use in pharmaceutical and other research around the world. His work has earned numerous awards and is well known among specialists in the field, yet the products are now so commonplace that they’re often used without any conscious attribution to Okamoto. Meidai’s University Professor Ryoji Noyori, who won the Nobel Prize in Chemistry in 2001, celebrates the attention the Japan Prize is drawing to Okamoto. “Many researchers around the world are indebted to Prof. Okamoto’s work,” he says.
 University Professor Yoshio Okamoto gives a speech at the Japan Prize award ceremony (photos and illustrations courtesy of: The Japan Prize Foundation)
University Professor Yoshio Okamoto gives a speech at the Japan Prize award ceremony (photos and illustrations courtesy of: The Japan Prize Foundation)
The motivation for awarding Prof. Okamoto the prize was due to his “leading contributions to precision synthesis of helical polymers and development of practical chiral materials for separating chiral drugs.
Here’s what that means in simpler terms. There is a category of molecules called “enantiomers” that, even though their chemical compositions are exactly the same, cannot be superimposed on each other and instead are configured as mirror images, like the right and left hand, a property known as “chirality” (see figure below). Interestingly, the effects of these molecules on living organisms differ depending on whether they are right or left-handed. Menthol, which gives off the characteristic mint aroma, and monosodium glutamate, which induces an umami flavor, are both examples of enantiomers. Many pharmaceuticals are also enantiomers, only one hand of which has a therapeutic effect. Nonetheless, standard chemical synthesis of these molecules could only produce mixtures of enantiomers, right and left.
Similarly, helical polymers when synthesized produced half-and-half mixtures of right and left-handed helix. Okamoto was the first in the world to succeed in synthesizing helical polymers of only one direction, also known as asymmetric synthesis. He also demonstrated a simple way to separate enantiomeric mixtures using such polymers. For example, from menthol, he was able to extract only the enantiomer that gave off the mint aroma. Based on these findings, companies commercialized separation instruments. Such instruments were deeply needed for research and production of everything from pharmaceuticals to aroma chemicals, and so were rapidly adopted around the world.
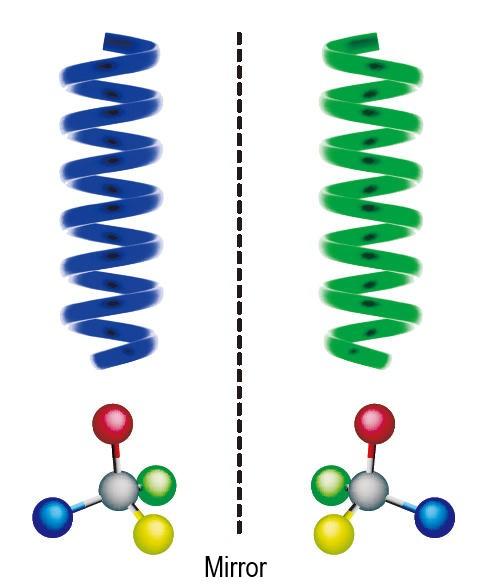 Enantiomers in a mirror-image relationship (below) and right and left-handed helical polymers (above)
Enantiomers in a mirror-image relationship (below) and right and left-handed helical polymers (above)
But according to Okamoto, such applications were not the initial aim of his research.
Helical polymers are molecules strung together in the shape of a helix. Many of the organic polymers that serve critical, life-sustaining functions, such as DNA known for its double helix structure and proteins, are helical, and these exist only in their right-handed forms. The ability to distinguish between right and left turns out to be a significant characteristic of life.
Okamoto decided to try synthesizing a helical polymer of only one direction. It didn’t go well. Just as he was about to give up, however, he was casually reading an American chemistry book when he learned of a certain reagent. The book was describing research Noyori had conducted at Kyoto University alongside his mentor Prof. Hitoshi Nozaki and others, and the reagent was used in that research. It was accessible and inexpensive, so Okamoto immediately purchased some. When he tried it in his lab, it worked much better than expected. He even “wondered if there was some kind of mistake,” he says. That was in 1979, when he was still an assistant at the Osaka University School of Engineering Science.
This alone was a world-first achievement worth celebrating, but then he wondered how he could use it. From a nearby lab doing research on organic synthesis he received a mixture of right and left-handed enantiomers and, passing them through a column packed with this polymer, found that one flowed out first, easily separating the two. The same-handed enantiomers caught hold of each other, much like shaking hands, whereas the left and right-handed enantiomers couldn’t, causing them to slip through and flow out first (see figure below). Even only slight differences, like a light handshake where only the finger tips catch, caused the two to separate beautifully. For each substance, Okamoto still couldn’t predict which would flow out first, nor did he fully understand the mechanism of separation. Apparently, the world of right and left is deep.
“Living organisms, too, must have a way of distinguishing slight differences,” says Okamoto.
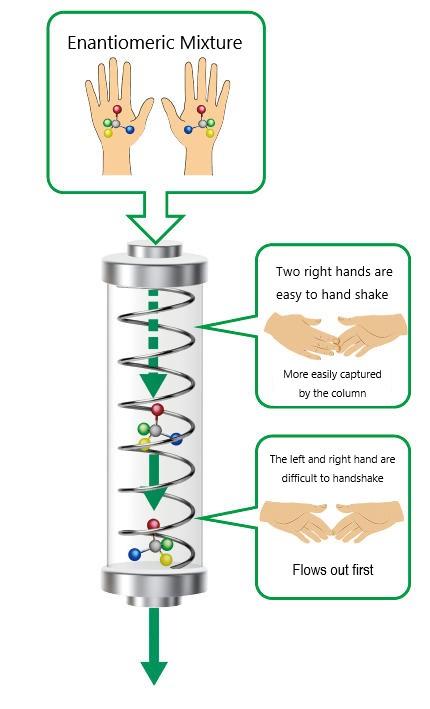 Mechanism of separating enantiomers in a helical polymer column
Mechanism of separating enantiomers in a helical polymer column
When Okamoto published his results in 1980, the response was huge. Just around that time, Noyori and other organic chemists were attempting asymmetric synthesis and were struggling to separate enantiomers to confirm their results. Requests to separate mixtures flowed in from researchers around the world, and Okamoto separated each of these in turn, publishing the results as a coauthored paper. He also contacted Noyori, who had transferred to Meidai, and separated the mixtures he made. Those results contributed to Noyori’s Nobel Prize.
Hence Noyori’s expression of gratitude toward Okamoto. Considering that it was Noyori’s research that catalyzed Okamoto’s rising success, the good fortune apparently went both ways.
Commercialization happened in 1982. Chemical company Daicel Corporation made a separating column by packing a tube with Okamoto’s helical polymer adsorbed to silica gel. As far as basic research at a university goes, the results were commercialized in an astonishingly short period. This was partly because Daicel had connections with a senior professor in the laboratory and employed its graduates, and also they were confident that there was demand.
The patent was acquired by Daicel. At the time, universities still had no industry collaboration framework and hadn’t yet formed any agreements. On the other hand, he received financial assistance for his research from Daicel, an arrangement that continues to this day. Patent income has been generated for the university since the creation of a patent management system.
Okamoto became a professor at Meidai in 1990, and since 2007, following mandatory retirement, has held a laboratory as Chair Professor at Harbin Engineering University in China. Although around 90% of substances with an enantiomer can now be separated, Okamoto continues to do research with the hopes of raising that number further and creating larger-scale separation systems.
 Prof. Okamoto holds a separating column containing helical polymers. Passing an enantiomeric mixture through the column separates them.
Prof. Okamoto holds a separating column containing helical polymers. Passing an enantiomeric mixture through the column separates them.
To be sure, the Japan Prize is a major achievement that pays high honor to “leading contributions.” But because Okamoto’s products have become so commonplace, their origins are largely forgotten. It may be the price for his huge success. Okamoto himself seems perfectly content, but one person who finds this irritating is Eiji Yashima, Professor at Meidai’s Graduate School of Engineering. As the foremost researcher of the synthesis and functions of helical polymers, Yashima says he stresses Okamoto’s contributions at every opportunity, especially at overseas conferences. Having been inspired by Okamoto’s work since he was a student at Osaka University, he eventually followed Okamoto to Meidai. “I hope the recent award will lead to a broader recognition of Prof. Okamoto’s achievements.”
Makoto Asashima, Professor Emeritus at the University of Tokyo and chair of the Japan Prize Selection Committee, is another person. “Prof. Okamoto’s contributions are extraordinary and I’m happy the Japan Prize has shed new light on them,” he says.
The Japan Prize is an international award given in the fields of science and technology. It was created in 1985 with a donation from Panasonic Corporation founder Konosuke Matsushita with the intent of creating a globally prestigious award, on par with the Nobel Prize, for “expressing Japan’s gratitude to international society.” Two recipients are chosen annually, with an individual cash prize of 50 million yen (around 460,000 US dollars). It’s managed by the Japan Prize Foundation (previously named the Science and Technology Foundation of Japan), launched with an endorsement from the prime minister’s cabinet. The presentation ceremony is held every April, with the Japanese emperor and empress in attendance. The name indicates its standing as an international award representing Japan.
The unfortunate truth, however, is that the Japan Prize lags far behind the Nobel Prize in terms of name recognition. News coverage is on a different order of magnitude. One attendee from outside Japan was surprised to notice how, despite the formality of the award ceremony, hardly any mention was made in the next day’s newspapers.
In an interview, Nobel Prize laureate Hideki Shirakawa concurred: “Japan makes too big a deal of the Nobel Prize. Japan has its own awards — the Japan Prize, the Kyoto Prize and so on — all wonderful, and yet they get only small articles. The difference is too great.”
More attention should be paid to these awards. Each award has its own assessment criteria, and the more diversity the better. There are also many fields that aren’t eligible for the Nobel Prize, such as mathematics.
Regarding this year’s Japan Prize, Asashima asks that attention also be given to the other laureate, Prof. Rattan Lal. An agricultural scientist from India, Lal has made significant contributions through soil research. Although there is the example of Norman Borlaug, agronomist and leader of the Green Revolution, winning the Nobel Peace Prize, agriculture itself is not a field eligible for science-related Nobel Prizes. Yet it goes without saying that agriculture has a critical role to play in solving the humanitarian issue of food security.
At the same time, opinion varies between awards. In 2014, when Okamoto won the Japan Academy Prize, it was Isamu Akasaki, inventor of the blue LED, who won the Imperial Prize and the Japan Academy Prize. Akasaki later won the Nobel Prize together with Hiroshi Amano and Shuji Nakamura, but it was only Akasaki who won the 2009 Kyoto Prize. The same three Nobel laureates had been presented the Takeda Award in 2002. Another interesting side note regarding the Nobel Prize given for the blue LED: some people argued for the contribution of Nick Holonyak, who invented the LED itself; Holonyak was awarded the Japan Prize in 1995. This shows how decisions about whom to honor vary greatly between awards for the same achievements. And there’s a reason behind each selection, which goes to show how many people made various contribution. These are connections you begin to make when you look across different awards.
Reflecting on his life as a researcher, Okamoto says, “Research was fascinating because I got results I didn’t expect, and because there was a runup period before I got there.” As advice to young researchers, he suggests, “If you give it everything you’ve got, you’ll come really close to a major discovery. Taking that last step requires years of honing your information receptors, and conviction.” Given the increasing difficulties young researchers face, Okamoto wants to help. He donated his Japan Prize money to Meidai, which has created an award for young researchers in his name. At the same time, I hope Meidai will do more to provide young researchers with the environment to do their research freely.

 Subscribe to RSS
Subscribe to RSS